Catalysis
| A catalyst is a substance which alters the rate of a chemical
reaction but is chemically unchanged at the end of the reaction. When two
different molecules collide with each other, they might react to make new
chemicals. How fast a chemical reaction is depends upon how frequently the
molecules collide. Catalysts increase the chance of molecules colliding. There
are two ways in which catalysts work:
| ||
|
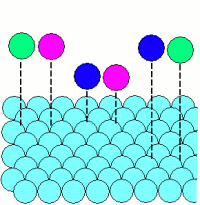
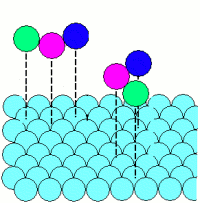
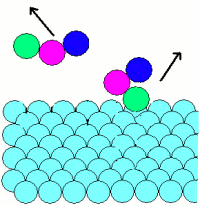
Adsorption: |
|
|
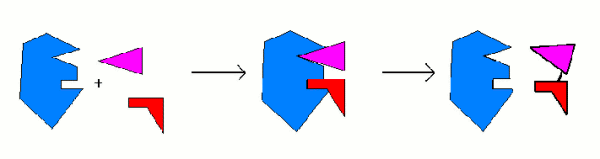
Intermediate Compounds:
Many catalysts, including all enzymes work by
forming intermediate compounds. The chemicals involved in the reaction combine
with the catalyst, making an intermediate compound. This new compound is very
unstable. When the intermediate compound breaks down, it releases the new
compounds and the original catalyst.